Subcutaneous Biologics Market Size Grows at 11.09% CAGR, Driven by Rising Demand for Self-Administration Therapies
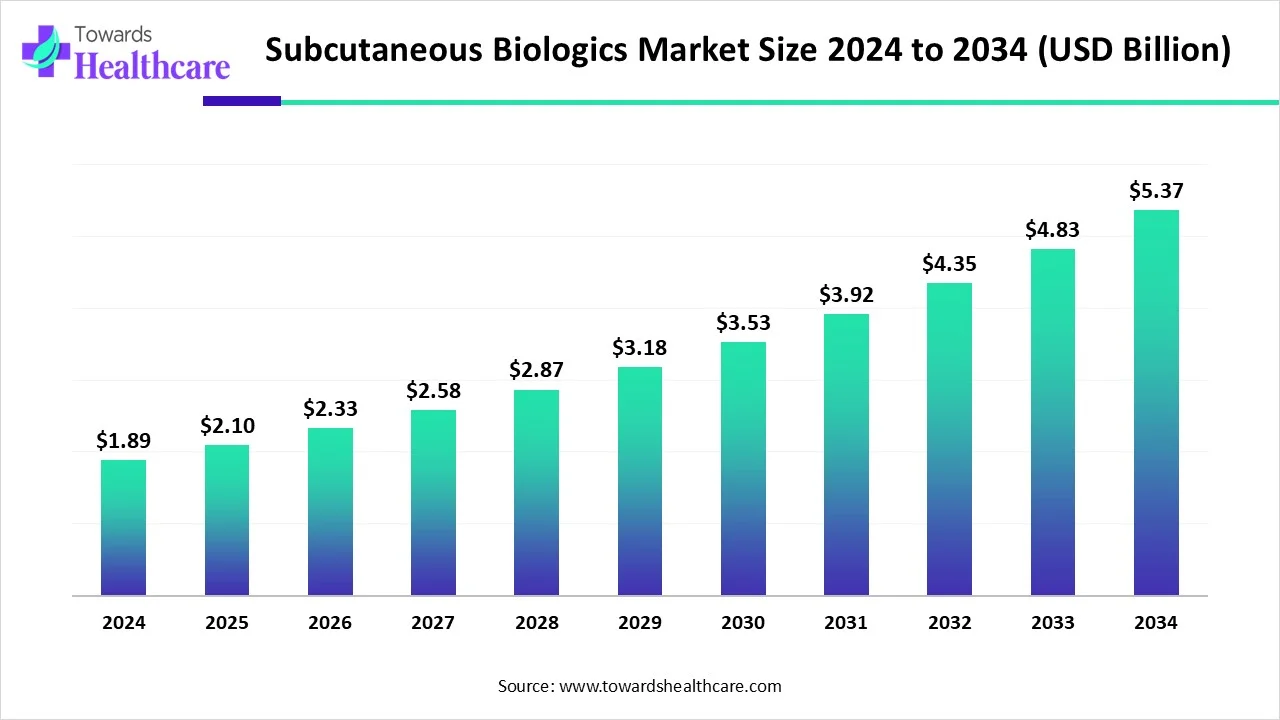
The subcutaneous biologics market size is calculated at USD 2.1 billion in 2025 and is expected to reach around USD 5.37 billion by 2034, growing at a CAGR of 11.09% for the forecasted period.
Ottawa, Oct. 28, 2025 (GLOBE NEWSWIRE) -- The global subcutaneous biologics market size was valued at USD 1.89 billion in 2024 and is predicted to hit around USD 5.37 billion by 2034, rising at a 11.09% CAGR, a study published by Towards Healthcare a sister firm of Precedence Research.
The global subcutaneous biologics market is rising due to growing demand for patient-friendly self-administrable therapies and the shift toward biologic drugs with targeted mechanisms of action.
The Complete Study is Now Available for Immediate Access | Download the Sample Pages of this Report @ https://www.towardshealthcare.com/download-sample/5726
Key Takeaways:
- North America dominated the global subcutaneous biologics market share in 2024.
- Asia-Pacific is expected to grow at the fastest CAGR in the market during the forecast period.
- By biologic type, the antibodies segment held a major revenue share of the market in 2024.
- By biologic type, the nucleotides segment is expected to expand rapidly in the market in the coming years.
- By administration method, the injection segment contributed to the biggest revenue share of the market in 2024.
- By administration method, the infusion segment is expected to show lucrative growth in the market over the studied period.
- By therapeutic area, the autoimmune disorders segment registered its dominance over the global subcutaneous biologics market in 2024.
- By therapeutic area, the oncological disorders segment is expected to show the fastest CAGR over the forecast period.
Market Overview:
What is propelling the growth of the Subcutaneous Biologics Market?
The global subcutaneous biologics market includes all biologic therapeutic agents (antibodies, nucleotides, proteins) delivered by subcutaneous (under-the-skin) route, in addition to drug-delivery technologies which enable subcutaneous delivery. Market growth can be attributed to various reasons such as rising chronic disease prevalence (autoimmune, oncology, metabolic), increasing convenience of outpatient/home use products, and advancements in formulations and delivery systems which have made the subcutaneous route more convenient and more appealing then the IV route. The subcutaneous route also reduces burden on infusion processes for providers and enhances the patients convenience while also providing companies the ability to create differentiated product offerings.
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
Key Metrics and Overview
| Metric | Details | |
| Market Size in 2025 | USD 2.1 Billion | |
| Projected Market Size in 2034 | USD 5.37 Billion | |
| CAGR (2025 - 2034) | 11.09 | % |
| Leading Region | North America | |
| Market Segmentation | By Biologic Type, By Administration Method, By Therapeutic Area, By Region | |
| Top Key Players | AbbVie, Inc., Biocon Biologics Ltd., Biogen, Inc., Eisai Co. Ltd., F. Hoffman-La Roche, Genentech, Inc., Lindy Biosciences, Merck, Novartis Pharma AG, Pfizer, SCHOTT Pharma, Takeda Pharmaceuticals | |
Major Growth Drivers:
Which key drivers are impacting the subcutaneous biologics market?
- The rising prevalence of autoimmune disorders, cancer indications and rare diseases around the world is increasing the number of biologic treatment options. Subcutaneous biologics offers a more appealing modality to deliver these therapies supporting market growth.
- Patients and healthcare systems continue to shift to medications that can be administered outside of institutional settings minimizing time and cost. Subcutaneous biologics combined with autoinjectors and/or pre-filled syringes are easy to administer at home or as an outpatient, improving adherence and market growth.
- Innovative delivery systems have improved the administration and delivery of biologics using improvements in high-concentration biologic formulation, wearable injectors, on-body patches and smart monitoring devices. These technological advances mitigate historical aspects of biological injection including viscosity and injection ease of use improving the potential for subcutaneous administration.
- Large pharmaceutical and biotechnology companies are investing heavily in developing and partnering on subcutaneous biologics continue to feed the advanced pipeline of new target products. The competitive nature and increase in approvals for subcutaneous biologics has provided great encouragement of manufacturers and investors to continue to explore this market and fast-track growth.
Get the latest insights on life science industry segmentation with our Annual Membership: https://www.towardshealthcare.com/get-an-annual-membership
Key Drifts:
- As manufacturers look to improve patient convenience, minimize infusion-centre congestion and utilize differentiation opportunities, the shift from intravenous administration to subcutaneous administration has gained prominence.
- Wearable and connected delivery devices are emerging as a platform for smart dose reminders, patient adherence reminders in the home or community, all of which is particularly important to chronic disease management.
- Biologics in higher-concentration formulations that allow for reduced injection volume are on the rise, helping to overcome technical challenges impacting prior subcutaneous delivery of large molecules.
Significant Challenge: Regulatory Challenges to Create Hurdles
Regulatory issues, formulation concerns, and immunogenicity challenges. There are several technical and regulatory issues in creating biologics for subcutaneous delivery. High molecular-weight biologics often face formulation challenges (e.g., viscosity, stability, bioavailability), and subcutaneous delivery can create more concerns around immunogenicity compared to intravenous delivery. The regulatory pathway does not get any easier either; novel delivery devices can complicate the regulatory process, while product recalls or adverse events could create negative perception and decrease market confidence.
Become a valued research partner with us - https://www.towardshealthcare.com/schedule-meeting
Regional Analysis:
North America is the leading region for subcutaneous biologics, which can be attributed to mature healthcare infrastructure, a high prevalence of chronic and autoimmune diseases, and emergence of biologic-drug manufacturers. Reports show the region held the highest revenue share in 2024 and forecast to remain dominant through the forecast period. Subcutaneous biologics are also supported by high R&D investments, favourable reimbursement systems and rapid uptake of home-use treatment formats. The U.S. also has a high concentration of large biopharmaceutical companies that can further support launch and scaling of subcutaneous biologics.
The Asia-Pacific region is emerging as fastest growing segment for subcutaneous biologics due to increased healthcare expenditures, increasing rates of chronic disease, rising patient awareness, and expanding biopharma activity in countries like China, India and Japan. The large untreated or under-treated population, favourable government initiative and improving health care infrastructure further wraps strong growth potential for subcutaneous biologic products. Reports indicate that Asia-Pacific is expected to register the highest CAGR among regions in the forecast period. The region further benefits from local manufacturing, increasing licensing partnerships and growth of biologics pipelines in region.
Download the single region market report @ https://www.towardshealthcare.com/checkout/5726
Segmental Insights:
By Biologic Type:
In 2024, the antibodies segment represented a substantial revenue share of the subcutaneous biologics market. Antibodies (i.e., monoclonal antibodies) are the most established biologic type used across the autoimmune, oncology and inflammatory indication markets and are increasingly being used in a subcutaneous formulation to promote ease of use and adherence. Multiple approved antibody biologics currently exists and are established in routine practice leading to a sustained presence.
The nucleotides segment (i.e., siRNA, antisense oligonucleotides) is expected to rapidly grow in the market in the coming years. Advancements in the gene- and nucleotide-based therapies, and formulation/delivery enhancements specifically for subcutaneous administration, will ensure that this segment grows quickly. With the advancement of the technology and approved nucleotide therapies for subcutaneous delivery, there is an opportunity for sustained high-growth in this segment.
By Administration Method:
Injection category is the largest revenue contributor to the market in 2024 (by administration type). Subcutaneous injection is the main route for therapeutics to be administered in an outpatient or self-administration setting, and typically has the highest value of sales. The combination of relatively simple logistics to manage, direct access to the patient, and alignment with home-care models helps the injection category remain the strongest revenue contributor.
Infusion category is expected to have significant growth opportunities in the studied period of the market. Infusion has historically been the main route for many biologics, but new hybrid options (infusion to subcutaneous, or infusion for loading and then subcutaneous for maintenance) and the movement of some therapies from infusion to subcutaneous (or mixed) means infusion still has large opportunities for growth. Some large molecule biologics will continue to require infusion, so infusion will remain relevant.
By Therapeutic Area:
By therapeutic area, the autoimmune disorders segment achieved its dominance of the global subcutaneous biologics market in 2024. Many of the earliest subcutaneous biologic therapies (for rheumatoid arthritis, psoriasis, Crohn’s disease) were in autoimmune and inflammatory indications, and the large patient base, broad adoption, and demand for self-administrable formats all support this dominance.
By therapeutic area, the oncological disorders segment is forecast to appear the fastest CAGR over the forecast period. An influx of oncology biologics reformulated for subcutaneous delivery, an increase in the global incidence of cancer, and a drive to lessen infusion-centre burden in oncology settings all contribute to the rapid growth of the oncology segment.
Browse More Insights of Towards Healthcare:
The global veterinary biological products market was valued at USD 17.9 billion in 2024, is expected to rise to USD 18.58 billion in 2025, and further reach around USD 25.9 billion by 2034, expanding at a CAGR of 3.78% between 2025 and 2034.
The biological sample temperature-controlled storage services market is witnessing steady momentum and is expected to generate significant revenue growth, potentially reaching hundreds of millions of dollars during the forecast period from 2025 to 2034.
The global biological therapies market was valued at USD 458.47 billion in 2024, increased to USD 501.71 billion in 2025, and is projected to reach approximately USD 1,107.66 billion by 2034, registering a CAGR of 9.35% between 2025 and 2034.
The global biological inactivated vaccine market was valued at USD 0.95 billion in 2024, grew to USD 1.00 billion in 2025, and is anticipated to reach around USD 1.57 billion by 2034, expanding at a CAGR of 5.14% from 2025 to 2034.
The global biologics safety testing market was valued at USD 4.03 billion in 2024, expanded to USD 4.58 billion in 2025, and is expected to reach around USD 14.45 billion by 2034, registering a strong CAGR of 13.64% between 2025 and 2034.
The filtration in biologics market is expected to experience robust growth from 2024 to 2034, fueled by the rising global demand for monoclonal antibodies, vaccines, and cell and gene therapies.
The global biological and chemical indicators market was valued at USD 570.67 million in 2024, increased to USD 600 million in 2025, and is forecast to reach approximately USD 942.03 million by 2034, growing at a CAGR of 5.14% from 2025 to 2034.
The global biologics contract research organization (CRO) market was valued at USD 31.15 billion in 2024, is projected to grow to USD 35.22 billion in 2025, and further reach USD 106.28 billion by 2034, driven by the rising outsourcing of R&D activities.
The global biologics CDMO market was valued at USD 22 billion in 2024, expanded to USD 25.41 billion in 2025, and is projected to hit around USD 92.79 billion by 2034, growing at a CAGR of 15.48% between 2025 and 2034.
The global biological PCR technology market was valued at USD 13.69 billion in 2023 and is expected to grow to USD 28.93 billion by 2034, registering a CAGR of 7.04% from 2024 to 2034.
Recent Developments:
- On 19 September 2025, Keytruda (pembrolizumab) from Merck & Co. was approved by the U.S. FDA for a subcutaneous formulation branded as Keytruda Qlex, enabling delivery in one to two minutes under the skin instead of a 30-minute IV infusion.
Subcutaneous Biologics Market Key Players List:

- AbbVie, Inc.
- Biocon Biologics Ltd.
- Biogen, Inc.
- Eisai Co. Ltd.
- F. Hoffman-La Roche
- Genentech, Inc.
- Lindy Biosciences
- Merck
- Novartis Pharma AG
- Pfizer
- SCHOTT Pharma
- Takeda Pharmaceuticals
Download the Competitive Landscape market report @ https://www.towardshealthcare.com/checkout/5726
Segments Covered in the Report
By Biologic Type
- Antibodies
- Nucleotides
- Proteins
By Administration Method
- Injection
- Infusion
- Both Injection & Infusion
By Therapeutic Area
- Autoimmune Disorders
- Bone Diseases
- Hematological Disorders
- Infectious Diseases
- Inflammatory Disorders
- Metabolic Disorders
- Neurological Disorders
- Ocular Disorders
- Oncological Disorders
- Others
By Region
- North America
- U.S.
- Canada
- Asia Pacific
- China
- Japan
- India
- South Korea
- Thailand
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Sweden
- Denmark
- Norway
- Latin America
- Brazil
- Mexico
- Argentina
- Middle East and Africa (MEA)
- South Africa
- UAE
- Saudi Arabia
- Kuwait
Immediate Delivery Available | Buy This Premium Research @ https://www.towardshealthcare.com/checkout/5726
Access our exclusive, data-rich dashboard dedicated to the healthcare market - built specifically for decision-makers, strategists, and industry leaders. The dashboard features comprehensive statistical data, segment-wise market breakdowns, regional performance shares, detailed company profiles, annual updates, and much more. From market sizing to competitive intelligence, this powerful tool is one-stop solution to your gateway.
Access the Dashboard: https://www.towardshealthcare.com/access-dashboard
About Us
Towards Healthcare is a leading global provider of technological solutions, clinical research services, and advanced analytics, with a strong emphasis on life science research. Dedicated to advancing innovation in the life sciences sector, we build strategic partnerships that generate actionable insights and transformative breakthroughs. As a global strategy consulting firm, we empower life science leaders to gain a competitive edge, drive research excellence, and accelerate sustainable growth.
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
Europe Region: +44 778 256 0738
North America Region: +1 8044 4193 44
APAC Region: +91 9356 9282 04
Web: https://www.towardshealthcare.com
Our Trusted Data Partners
Precedence Research | Statifacts | Towards Packaging | Towards Automotive | Towards Food and Beverages | Towards Chemical and Materials | Towards Consumer Goods | Towards Dental | Towards EV Solutions | Nova One Advisor | Healthcare Webwire | Packaging Webwire | Automotive Webwire | Nutraceuticals Func Foods | Onco Quant | Sustainability Quant | Specialty Chemicals Analytics
Find us on social platforms: LinkedIn | Twitter | Instagram | Medium | Pinterest

Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.

